Answer:
The concentration of hydrogen ion at pH is equal to 2 :
![= [H^+]=0.01 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/gbidrdxadkacog8o17ytdb208ny4d2c90q.png)
The concentration of hydrogen ion at pH is equal to 6 :
![[H^+]'=0.000001 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/uo68iy9xhgdzahzh34opi9427iouqphpa7.png)
There are 0.009999 more moles of
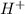 ions in a solution at a pH = 2 than in a solution at a pH = 6.
ions in a solution at a pH = 2 than in a solution at a pH = 6.
Step-by-step explanation:
The pH of the solution is the negative logarithm of hydrogen ion concentration in an aqueous solution.
![pH=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/d4u8c7rky5aqengst85apsbxbk128yl4er.png)
The hydrogen ion concentration at pH is equal to 2 = [H^+]
![2=-\log [H^+]\\](https://img.qammunity.org/2022/formulas/physics/high-school/d2z8i3mmcw92qv6tjebv1ylrsx86vce64e.png)
![[H^+]=10^(-2)M= 0.01 M=0.01 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/prvvixh8qdgo3t6tyrvbx3vumcih18qv3n.png)
The hydrogen ion concentration at pH is equal to 6 = [H^+]
![6=-\log [H^+]\\\\](https://img.qammunity.org/2022/formulas/physics/high-school/j8kq7l22ym16err5rdru2exsjjv23zhog1.png)
![[H^+]=10^(-6)M= 0.000001 M= 0.000001 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/gv9q39dpsjjulid3iib208n7bpavnmmnsv.png)
Concentration of hydrogen ion at pH is equal to 2 =
![[H^+]=0.01 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/cbjqduqv60hihmi9jcdx2z6kwchc3v841a.png)
Concentration of hydrogen ion at pH is equal to 6 =
![[H^+]'=0.000001 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/uo68iy9xhgdzahzh34opi9427iouqphpa7.png)
The difference between hydrogen ion concentration at pH 2 and pH 6 :
![= [H^+]-[H^+]' = 0.01 mol/L- 0.000001 mol/L = 0.009999 mol/L](https://img.qammunity.org/2022/formulas/physics/high-school/w7okndoqsnkjdyjtlzbdtiqklycb7osmql.png)
Moles of hydrogen ion in 0.009999 mol/L solution :
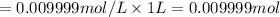
There are 0.009999 more moles of
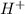 ions in a solution at a pH = 2 than in a solution at a pH = 6.
ions in a solution at a pH = 2 than in a solution at a pH = 6.