Answer:
The percentage yield of LiCl is approximately 75.365%
Step-by-step explanation:
The given chemical reaction is presented as follows;
LiOH + KCl → LiCl + KOH
1 ole of LiOH reacts with 1 mole of KCl to produce 1 mole of LiCl and 1 mole of KOH
The mass of LiOH in the reaction = 5.00 grams
The mass of KCl in the reaction = 3.50 grams
The mass of LiCl produced in the reaction = 1.50 grams
The number of moles, n = Mass/(Molar mass)
The molar mass of LiOH = 23.95 g/mol
The number of moles of LiOH in the reaction, n = 5.00 g/(23.95 g/mol) ≈ 0.209 moles
The molar mass of KCl = 74.5513 g/mol
The number of moles of KCl in the reaction, n = 3.50 g/(74.5513 g/mol) ≈ 0.0469475281 moles
The molar mass of LiCl = 42.394 g/mol
The number of moles of LiCl produced, n = 1.50 g/(42.394 g/mol) ≈ 0.035382 moles
Therefore, the limiting reactant = KCl with 0.0469475281 moles
We get;
0.0469475281 moles of LiOH should react with 0.0469475281 moles of KCl to procude 0.0469475281 moles of LiCl and 0.0469475281 moles of KOH
The percentage yield is given as follows;
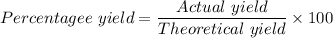
The actual yield of LiCl ≈ 0.035382 moles of LiCl
The theoretical yield of LiCl ≈ 0.0469475281 moles of LiCl
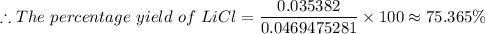
The percentage yield of lithium chloride, LiCl ≈ 75.365%.