Answer:
These atoms belong to the same element (sulfur, atomic number
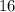 .) Hence, the atomic number of each atom would be equal to the atomic number of sulfur:
.) Hence, the atomic number of each atom would be equal to the atomic number of sulfur:
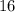 .
.
Each of these isotopes contains a different number of neutrons. Hence, the mass number (number of neutrons and protons in the atom) differ.
Step-by-step explanation:
The atomic number of an atom is the number of protons in the atom.
The mass number of an atom is the number of protons in this atom, plus the number of neutrons in this atom.
The exact element (e.g., sulfur) that an atom belongs to depends on the atomic number of that atom. For example, an atom with atomic number
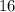 belongs to sulfur, while an atom with atomic number
belongs to sulfur, while an atom with atomic number
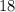 belongs to argon.
belongs to argon.
All four types of atoms in this sample belongs to the element sulfur. The atomic number of sulfur is
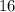 . Hence, the atomic number of all four types of atoms must also be
. Hence, the atomic number of all four types of atoms must also be
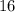 . Otherwise, these atoms would belong to other elements and won't be named after "sulfur".
. Otherwise, these atoms would belong to other elements and won't be named after "sulfur".
Hence, all atoms in this sample would have the same atomic number:
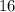 . Each of these atoms would contain
. Each of these atoms would contain
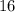 protons.
protons.
While the number of protons in each of these atoms is fixed, the number of neutrons in each of these atoms could still vary. Because the mass number of an atom accounts for both protons and neutrons, the mass number of these atoms would not be the same.
For example, sulfur-
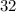 (mass number
(mass number
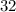 ) contains
) contains
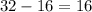 neutrons, whereas sulfur-
neutrons, whereas sulfur-
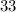 (mass number
(mass number
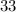 ) contains
) contains
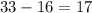 neutrons. (The
neutrons. (The
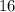 in the left-hand side is the number of protons in each of these atoms.) Hence, these types of atoms have different mass numbers even though they share the same proton number.
in the left-hand side is the number of protons in each of these atoms.) Hence, these types of atoms have different mass numbers even though they share the same proton number.