Answer:
V = 0.104 m³
Step-by-step explanation:
Given that,
Number of moles, n = 7.70 moles
Pressure, P = 202.6 kPa
Temperature, T = 59.0°C = 332 K
We need to find the volume of the container that the gas. We know that the ideal gas law is as follows :
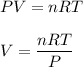 , R =8.314 m³-Pa/K-mol
, R =8.314 m³-Pa/K-mol
Put all the values,
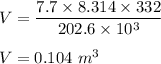
So, the volume of the container is equal to 0.104 m³.