Answer:
T = 486.6 K
Step-by-step explanation:
The final temperature of the block can be found using the following formula:
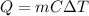
where,
Q = Thermal Energy Transferred = 3.35 x 10⁵ J
m = mass of aluminum block = 2 kg
C = Specific Heat = 897 J/kg.K
ΔT = Change in Temperature = T - 300 K
T = Final Temperature of the Block = ?
Therefore,
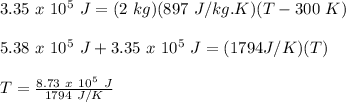
T = 486.6 K