Answer:
V = 0.5 L
Step-by-step explanation:
Given that,
The volume of HCl, V = 2 M
We need to find the volume of 2.0M HCl is needed to prepare 0.50L of a 0.75M solution.
Let n be the number of moles of HCl.
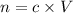
Where
c is molarity
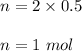
Let V be the volume of the solution. So,
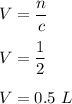
So, the required volume of the solution is equal to 0.5 L.