Answer:
The new concentration of the solution is 0.89 M.
Step-by-step explanation:
In chemistry, dilution is the reduction in concentration of a chemical in a solution. This is accomplished by adding more solvent to the same amount of solute.
So, in a dilution, the amount of solute does not vary, but the volume of the solvent varies: as more solvent is added, the concentration of the solute decreases, as the volume (and weight) of the solution increases.
In a dilution the expression is used:
Ci*Vi = Cf*Vf
where:
- Ci: initial concentration
- Vi: initial volume
- Cf: final concentration
- Vf: final volume
In this case:
- Ci= 2.5 M
- Vi= 250 mL
- Cf=?
- Vf= 700 mL
Replacing:
2.5 M* 250 mL= Cf* 700 mL
Solving:
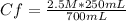
Cf= 0.89 M
The new concentration of the solution is 0.89 M.