Answer:
(I)
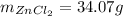
(II)
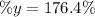
(III) The presence of impurities in the 32.70 g of zinc will support the student's percentage yield.
Step-by-step explanation:
The reaction is:
Zn + 2HCl → ZnCl₂ + H₂
(I) To find the number of moles of ZnCl₂ we need to calculate the number of moles of the reactants.
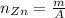
Where:
m: is the mass
A: is the standard atomic weight
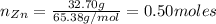
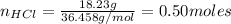
If 1 mol of Zn reacts with 2 moles of HCl, the number of moles of Zn needed to react with 0.50 moles of HCl is:
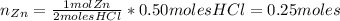
Then, the limiting reactant is HCl. Now, we can find the number of moles of ZnCl₂ by knowing that 2 moles of HCl produce 1 mol of ZnCl₂.
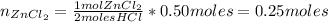
Finally, the mass of ZnCl₂ that he has produced is:
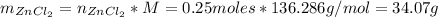
(II) The percentage yield can be found with the following equation:
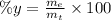
Where:
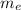 : is the experimental mass = 60.1 g
: is the experimental mass = 60.1 g
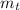 : is the theoretical mass = 34.07 g
: is the theoretical mass = 34.07 g
Hence, the percentage yield is:
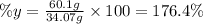
(III) The presence of impurities in zinc will explain why the percentage yield is greater than 100% because the mass of the impurities will make the mass of zinc bigger than its mass without impurities. If there were no impurities in the zinc, its mass would be smaller and thus the percent yield.
I hope it helps you!