Answer: The molality of given acetic acid solution is 0.625 m.
Step-by-step explanation:
Given: Moles of solute = 0.5 mol
Mass of solvent = 0.8 kg
Molality is the number of moles of solute present in a kg of solvent.
Therefore, molality of the given solution is calculated as follows.
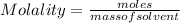
Substitute the values into above formula as follows.
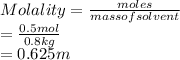
Thus, we can conclude that the molality of given acetic acid solution is 0.625 m.