Answer: (a) There are 0.428 moles present in 12 g of
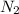 molecule.
molecule.
(b) There are 2 moles present in
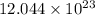 particles of oxygen.
particles of oxygen.
Step-by-step explanation:
(a). The mass of nitrogen molecule is given as 12 g.
As the molar mass of
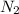 is 28 g/mol so its number of moles are calculated as follows.
is 28 g/mol so its number of moles are calculated as follows.
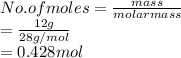
So, there are 0.428 moles present in 12 g of
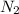 molecule.
molecule.
(b). According to the mole concept, 1 mole of every substance contains
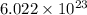 atoms.
atoms.
Therefore, moles present in
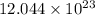 particles are calculated as follows.
particles are calculated as follows.
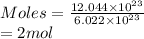
So, there are 2 moles present in
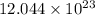 particles of oxygen.
particles of oxygen.