Answer:
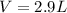
Step-by-step explanation:
Hello there!
In this case, by considering the given information in this problem, it is possible for us to infer that this problem is solved by using the ideal gas equation:

Next, since we are given the moles, pressure and temperature, we proceed as follows:
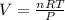
Then, we plug in the given data to obtain:

Best regards!