Answer:
New volume = 105.6 mL
Step-by-step explanation:
Given that,
Temperature, T = 240.0 K
Pressure, P = 670 mm Hg
Volume, V = 128 mL
New temperature = -75°C = 198 K
We need to find the new volume. Let it is V'. The relation between volume and the temperature is given by :
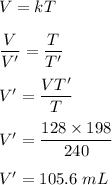
So, the new volume is equal to 105.6 mL.