Answer:
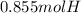
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to realize this problem is about mole ratios. Thus, for the compound C4H10, note there is a 1:10 mole ratio to the hydrogen atoms, that is why the number of moles of the latter is calculated as shown below:
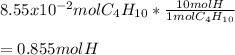
Regards!