Answer:
-0.21 if you're using C as your temperature.
0.018 if you're using K as your temperature.
Step-by-step explanation:
This is similar to your other question, where we'll be using the formula for specific heat capacity: Q=mcΔT
Q = heat energy (Joules)
m = mass (g)
c = specific heat capacity > What we're solving for.
ΔT = change in temperature > final temp. - initial temp.
It would be helpful to know the units so you'll know what type of problem you're dealing with and what formula you should use. But you'll usually use this formula if they mention the specific heat of an material.
Let's plug in what we know.
700= (150)(c)(32-54)
700= (150)(c)(-22) we want to solve for c, to make it easier let's multiply the numbers on the right side so we'll have...
700=-3300c
Solve for c.
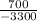 = c
= c
-0.21 = c
This is in C though, your depending on your teacher they might want you to convert C to Kelvin (K). So Here is what it would be if it was in Kelvin.
700= (150)(c)(251)
700=37650c
0.018=c