Answer:

Step-by-step explanation:
We are asked to convert from molecules of water to moles.
1. Avogadro's Number
1 mole of any substance contains the same number of particles (atoms, molecules, formula units, etc). This is Avogadro's Number: 6.022*10²³. In this problem, the particles are molecules of water.
2. Convert Molecules to Moles
Use Avogadro's Number to make a ratio.
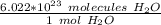
Multiply by the number of molecules given: 2.54 *10²⁹
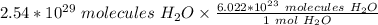
Flip the ratio so the units of molecules of water cancel.
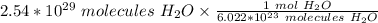
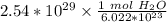
Condense the problem into 1 fraction.
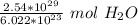
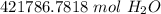
2.54 *10²⁹ molecules of water are 421, 786.7818 moles of water.