Answer:

Step-by-step explanation:
We need to use stiochiometry and a mole to mole conversion to solve this problem.
First, examine the chemical equation. Make sure it is balanced before doing any calculations.
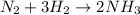
It is balanced, so we can also use the coefficients to refer to molar amounts.
So, the equation is also saying that 1 mole of N₂ (no coefficient implies 1) and 3 moles of H₂ react to form 2 moles of NH₃.
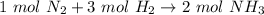
Now we can use this information to make a ratio. We know that we have 0.45 moles of hydrogen, and we are trying to find the moles of ammonia.
According to the original equation, 3 moles of hydrogen produce 2 moles of ammonia. Let's make a ratio.
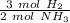
We have 0.45 moles of hydrogen, so multiply by that number.
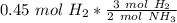
Flip the ratio so the units of moles of hydrogen cancel.
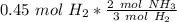
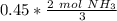
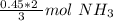
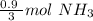
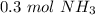
0.3 moles of ammonia are produced when 0.45 moles of hydrogen gas react with nitrogen gas.