Answer:
0.1 liters of NaOH.
Step-by-step explanation:
When the acid solution is neutralized we have:
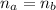
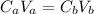
Where:
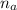 : is the number of moles of the acid
: is the number of moles of the acid
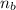 : is the number of moles of the base
: is the number of moles of the base
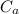 : is the concentration of the acid
: is the concentration of the acid
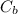 : is the concentration of the base = 0.1 M
: is the concentration of the base = 0.1 M
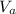 : is the volume of the acid = 10000 L
: is the volume of the acid = 10000 L
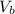 : is the volume of the base =?
: is the volume of the base =?
The concentration of the acid can be calculated from the pH:
![pH = -log([H^(+)])](https://img.qammunity.org/2022/formulas/chemistry/college/nd7uu1zdiir7bsf3i9tpwhne2qb6y4cfjb.png)
![[H^(+)] = 10^(-pH) = 10^(-6) M](https://img.qammunity.org/2022/formulas/chemistry/college/1nrjbq1yswkxzagdypsna92ya4rm114s56.png)
Now, we can find the volume of the base:
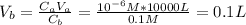
Therefore, the amount of NaOH needed to neutralize the solution is 0.1 liters.
I hope it helps you!