Answer:
The temperature of the sample increase by 48 Kelvin
Step-by-step explanation:
The sample is identical.
Hence the heat at constant pressure is equal to the heat at the constant Volume
Q1 = Q2
Q 1 = heat at constant pressure
Q2 = heat at the constant Volume
Substituting the given values, we get -
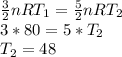
The temperature of the sample increase by 48 Kelvin