Answer:
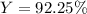
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction, it is possible for us to calculate the percent yield by dividing the actual yield of 143 grams of gaseous chlorine by the theoretical yield of 155 g of this gas. In such a way, we proceed as follows:
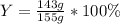
Which has a result of:
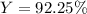
Regards!