Answer: The mass of lead deposited on the cathode of the battery is 1.523 g.
Step-by-step explanation:
Given: Current = 62.0 A
Time = 23.0 sec
Formula used to calculate charge is as follows.
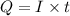
where,
Q = charge
I = current
t = time
Substitute the values into above formula as follows.
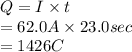
It is known that 1 mole of a substance tends to deposit a charge of 96500 C. Therefore, number of moles obtained by 1426 C of charge is as follows.
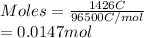
The oxidation state of Pb in
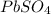 is 2. So, moles deposited by Pb is as follows.
is 2. So, moles deposited by Pb is as follows.
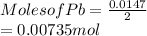
It is known that molar mass of lead (Pb) is 207.2 g/mol. Now, mass of lead is calculated as follows.
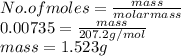
Thus, we can conclude that the mass of lead deposited on the cathode of the battery is 1.523 g.