Answer:
Step-by-step explanation:
From the given information:
Water freezing temp.
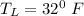
Heat rejected temp
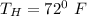
Recall that:
The coefficient of performance is:
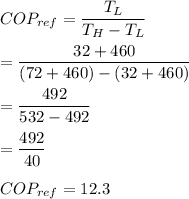
Again:
The efficiency given by COP can be represented by:
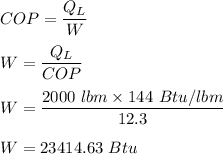
Finally; the power input in an hour can be determined by using the formula:

In hp; since 1 kW = 1.34102 hp
6.86kW will be = (6.86 × 1.34102) hp
= 9.199 hp