Solution :
Given :
Initial temperature of the refrigerant is :
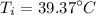
= ( 39.37 + 273 ) K
= 312.3 K
Room which is maintained at constant temperature is :
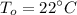
= (22+273) K
= 295 K
The thermal energy transferred to the room is :
Q = 400 kJ
=
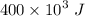
Therefore, the total entropy generation during the thermal energy process is :
![$\Delta S =\left[(-Q)/(T_i)+ (+Q)/(T_i)\right]$](https://img.qammunity.org/2022/formulas/chemistry/college/faigikcwc7w8uy6naguhsfw3avkd7rov1z.png)
Here, -Q = heat is leaving the system maintained at a temperature of
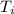 K.
K.
+Q = heat is entering the system maintained at a temperature of
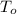 K.
K.
Therefore, substituting the values :
![$\Delta S =\left[(-400* 10^3)/(312.3)+ (400* 10^3)/(295)\right]$](https://img.qammunity.org/2022/formulas/chemistry/college/pehjpnzpb2t7irfgw7i31wxg01czy1t5l1.png)
= [-1280.8197 + 1355.9322]
= 75.1125 J/K
= 0.0751125 kJ/K
= 0.075 kJ/K