Answer:
![Ksp=([Co^(2+)][S^(2-)])/(1)](https://img.qammunity.org/2022/formulas/chemistry/college/re2kkbqzj5acil8m3rgbdi18xtxr97rzdl.png)
Step-by-step explanation:
Hello there!
In this case, according to the given information, it turns out firstly necessary to write the chemical equation for this reaction as shown below:
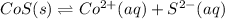
Thus, since solids are not included in equilibrium expressions, we can set this one up as follows:
![Ksp=([Co^(2+)][S^(2-)])/(1)](https://img.qammunity.org/2022/formulas/chemistry/college/re2kkbqzj5acil8m3rgbdi18xtxr97rzdl.png)
Regards!