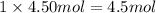Answer:
1. In 0.0250 moles of potassium chromate there are 0.05 moles of potassium , 0.025 moles of chromium and 0.1 moles of oxygen are present.
2. In 4.50 moles of ammonium carbonate there are 9 moles of ammonium ions and 4.5 moles of carbonate ions are present.
Step-by-step explanation:
1.
Moles of potassium chromate = 0.0250 mol
Formula of potassium chromate =
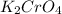
1 mole of potassium chromate has 2 moles of potassium , 1 mole of chromium and 4 moles of oxygen.
Then in 0.0250 moles of potassium chromate:
Moles of potassium will be:
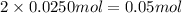
Moles of chromium will be:
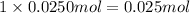
Moles of oxygen will be:
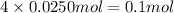
2.
Moles of ammonium carbonate = 4.50 mol
Formula of ammonium carbonate =
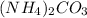
1 mole of ammonium carbonate has 2 moles of ammonium ion and 1 mole of carbonate ion.
Then in 4.50 moles of ammonium carbonate:
Moles of ammonium ion will be:
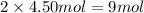
Moles of carbonate ions will be: