Answer:
The limiting reactant is propene,
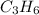 .
.
Step-by-step explanation:
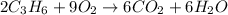
Moles of nitrogen propene = 2 mol
Moles of oxygen = 10 mol
According to reaction, 2 moles of propene reacts with 9 moles of oxygen gas, then 2 moles of propene will react with:
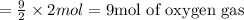
According to the question, we have 10 moles of oxygen gas, which is more than 9 moles of oxygen gas. This indicates that propene is present in a limiting amount hence, it is a limiting reactant.
The limiting reactant is propene, hence the correct answer is the
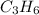 .
.