Answer:
![Ksp=[Ni^(2+)][CN^-]^2\\\\Ksp=[Ag^+][OH^-]](https://img.qammunity.org/2022/formulas/chemistry/college/rco95zpv0h8vkpb7av24b88glz5vuwamfj.png)
Step-by-step explanation:
Hello there!.
In this case, for this equilibrium problem, it turns out firstly necessary to write the chemical equations for the solubilization of both nickel (II) cyanide and silver hydroxide as shown below:
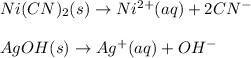
Thus, by means of the law of mass action we set up these equilibrium expressions as shown below:
![Ksp=[Ni^(2+)][CN^-]^2\\\\Ksp=[Ag^+][OH^-]](https://img.qammunity.org/2022/formulas/chemistry/college/rco95zpv0h8vkpb7av24b88glz5vuwamfj.png)
Best regards!