Answer:
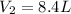
Step-by-step explanation:
Hello there!
In this case, according to the definition of the Boyle's law, which describes de pressure-volume behavior as an inversely proportional relationship, it is possible for us to write:
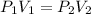
Thus, since we are given the initial pressure and temperature, and the final pressure, we are able to calculate the final volume as shown below:
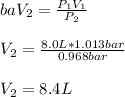
Regards!