Answer:
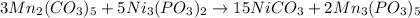
Step-by-step explanation:
Hello there!
In this case, for the given reactants side in this chemical reaction, it is possible for us to firstly write the left side of the undergoing chemical equation as shown below:
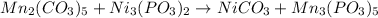
Which means that the products are nickel (II) carbonate and manganese (V) phosphite. Next, we balance the reaction as shown below:
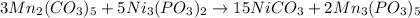
Which makes 15 carbonate ions, 10 phosphite ions, 15 nicked (II) ions and 6 manganese (V) on both sides of the chemical equation.
Best regards!