Answer:
At about 51 °C
Step-by-step explanation:
Hello there!
In this case, according to the attached solubility chart of potassium nitrate, our first step for this problem is to calculate the grams of this solute in 100 g given that 23 g are soluble in 25 g of water:
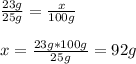
Thus, such solubility of 92 g in 100 g of water is exhibited at about 51 °C.
Regards!