Answer:
The half-life of the radioactive substance is of 3.25 days.
Explanation:
The amount of radioactive substance is proportional to the number of counts per minute:
This means that the amount is given by the following differential equation:
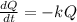
In which k is the decay rate.
The solution is:
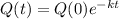
In which Q(0) is the initial amount:
8000 counts per minute on a Geiger counter at a certain time
This means that
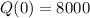
500 counts per minute 13 days later.
This means that
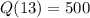 . We use this to find k.
. We use this to find k.
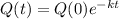
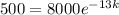
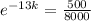
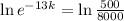
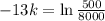
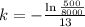
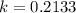
So
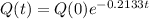
Determine the half-life of the radioactive substance.
This is t for which Q(t) = 0.5Q(0). So
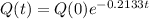
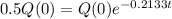
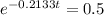
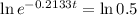
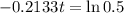
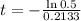
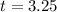
The half-life of the radioactive substance is of 3.25 days.