Answer:
(a) W = 3293 J = 3.293 KJ
(b) W = 0 KJ
(c) W = -506.6 J = -0.507 KJ
(d) W = 0 KJ
Net Work = 2.786 KJ = 2786 J
Step-by-step explanation:
(a)
The work done is given as:
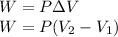
where,
P = Constant Pressure = (6.5 atm)(101325 Pa/1 atm) = 6.59 x 10⁶ Pa
V₁ = initial volume = (1 L)(0.001 m³/1 L) = 0.001 m³
V₂ = final volume = (6 L)(0.001 m³/1 L) = 0.006 m³
Therefore,
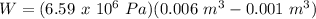
W = 3293 J = 3.293 KJ
(b)
Since the volume is constant in this stage. Therefore,
ΔV = 0
As a result:
W = 0 KJ
(c)
The work done is given as:
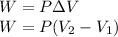
where,
P = Constant Pressure = (1 atm)(101325 Pa/1 atm) = 1.01 x 10⁵ Pa
V₁ = initial volume = (6 L)(0.001 m³/1 L) = 0.006 m³
V₂ = final volume = (1 L)(0.001 m³/1 L) = 0.001 m³
Therefore,
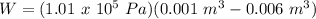
W = -506.6 J = -0.507 KJ
negative sign show that the work is done on the gas by the environment.
(d)
Since the volume is constant in this stage. Therefore,
ΔV = 0
As a result:
W = 0 KJ
Net work will be the sum of all the works:
Net Work = 3.293 KJ + 0 KJ - 0.507 KJ + 0 KJ
Net Work = 2.786 KJ = 2786 J