Answer:
ΔT = 0.29°C
Step-by-step explanation:
Using the law of conservation of energy:
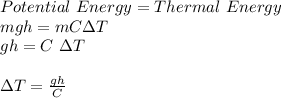
where,
ΔT = Temperature difference between the water at the top and the bottom of the fall = ?
g = acceleration due to gravity = 9.81 m/s²
h = height of fall = 125 m
C = specific heat capacity of water = 4200 J/kg
Therefore,
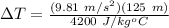
ΔT = 0.29°C