Answer:
See explanation.
Step-by-step explanation:
Hello there!
In this case, according to the described chemical reaction, we first write the corresponding equation to obtain:
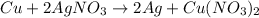
Thus, we proceed as follows:
Part 1 of 3: here, since the molar mass of silver and copper (II) nitrate are 107.87 and 187.55 g/mol respectively, and the mole ratio of the former to the latter is 2:1, we can set up the following stoichiometric expression:
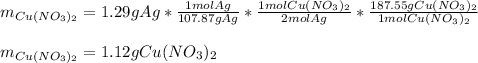
Part 2 of 3: here, the molar mass of copper is 63.55 g/mol and the mole ratio of silver to copper is 2:1, the mass of the former that was used to start the reaction was:
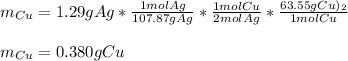
Part 3 of 3: here, the molar mass of silver nitrate is 169.87 g/mol and their mole ratio 2:2, thus, the mass of initial silver nitrate is:
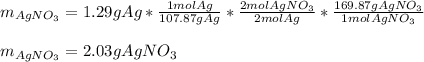
Best regards!