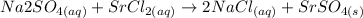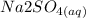 The balanced equation for the reaction of a solution of sodium sulfate is mixed with strontium chloride is:
The balanced equation for the reaction of a solution of sodium sulfate is mixed with strontium chloride is:
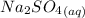
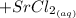
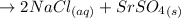
This is a precipitation reaction, which means that one of the products is a solid that precipitates out of solution. In this case, the solid product is strontium sulfate,
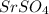 .
.
The balanced equation for the reaction of a solution of sodium sulfate mixed with strontium chloride is :
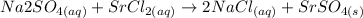 . This is a precipitation reaction, which means that one of the products is a solid that precipitates out of solution. In this case, the solid product is strontium sulfate,
. This is a precipitation reaction, which means that one of the products is a solid that precipitates out of solution. In this case, the solid product is strontium sulfate,
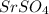
When a solution of sodium sulfate,
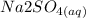 , is mixed with a solution of strontium chloride,
, is mixed with a solution of strontium chloride,
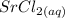 , a double displacement reaction occurs. In a double displacement reaction, the positive and negative ions of the two reactants switch partners to form two new products.
, a double displacement reaction occurs. In a double displacement reaction, the positive and negative ions of the two reactants switch partners to form two new products.
In this case, the sodium ions, Na+, from the sodium sulfate react with the chloride ions, Cl-, from the strontium chloride to form sodium chloride, NaCl. The strontium ions,
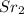 +, from the strontium chloride react with the sulfate ions,
+, from the strontium chloride react with the sulfate ions,
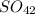 -, from the sodium sulfate to form strontium sulfate,
-, from the sodium sulfate to form strontium sulfate,
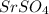 .
.
Strontium sulfate is a white, insoluble solid. This means that it will precipitate out of solution and form a white solid at the bottom of the reaction vessel.
This reaction can be used to identify the presence of strontium ions in a solution. If a solution of sodium sulfate is added to a solution of strontium ions and a white precipitate forms, then this indicates the presence of strontium ions in the solution.
Therefore, The balanced equation for the reaction is: