Answer:
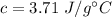
Step-by-step explanation:
Given that,
Mass of sample, m = 996.9 g
The change in temperature of the sample,
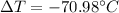
Heat produced, Q = 62.9 calories = 263173.6 J
The heat released by a sample due to change in temperature is given by :
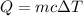
Where
c is the specific heat capacity
So,
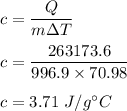
So, the specific heat of ethanol is equal to
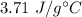 .
.