Answer:
P₂ = 1.22 kPa
Step-by-step explanation:
This problem can be solved using the equation of state:
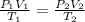
where,
P₁ = initial pressure = 1 KPa
P₂ = final pressure = ?
V₁ = initial Volume = 1 liter
V₂ = final volume = 1.1 liter
T₁ = initial temperature = 290 k
T₂ = final temperature = 390 k
Therefore,
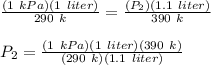
P₂ = 1.22 kPa