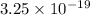Answer:
The mass of 1000 atoms of gold is, in grams, of
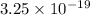
Explanation:
This question is solved by proportions, using a rule of three.
We have that:
One atom of gold has a mass, in grams, of
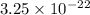 . What is the mass of 1000 atoms of gold?
. What is the mass of 1000 atoms of gold?
1000 atoms is
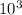 . So
. So
1 atom -
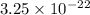 grams
grams
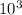 atoms - x
atoms - x
Applying cross multiplication:
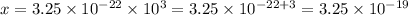
The mass of 1000 atoms of gold is, in grams, of