Answer:

Step-by-step explanation:
Molarity is concentration measured in moles per liters. It is the number of moles of solute per liters of solution. The formula is:
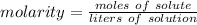
We know the solution of HCl has a molarity of 0.5 molar and there are 0.8 liters of solution.
- 1 molar (M) is equal to 1 mole per liter.
- Let's convert the molarity of 0.5 M HCl to 0.5 mol HCl per liter. This will make unit cancellation easier.
The moles of solute or HCl are unknown, so we can use x. Now, we can substitute all known values into the formula.
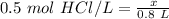
Since we are solving for the moles of solute (x), we must isolate the variable. It is being divided by 0.8 liters. The inverse of division is multiplication, so we multiply both sides by 0.8 L.
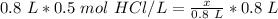
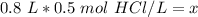
The units of liters (L) cancel.
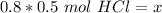
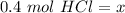
This solution contains 0.4 moles of HCl.