Answer:
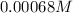
Step-by-step explanation:
Hello there!
In this case, according to the ionization of calcium hydroxide, a strong base:
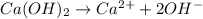
Thus, since there is a 1:2 mole ratio of calcium hydroxide to hydroxide ions, we apply the following proportional factor to obtain:
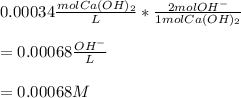
Regards!