Answer:
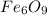
Step-by-step explanation:
Hello there!
In this case, since these problems about formulas, firstly require the determination of the empirical formula, assuming that the given percentages are masses, we can calculate the moles and mole ratio of oxygen to iron as shown below:
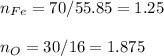
In such a way, by rounding to the first whole number we multiply by 8 and divide by 5 to obtain:
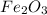
Whose molar mass is 159.69 g/mol and the mole ratio of the molecular to the empirical formula is:
479.1/159.69=3
Therefore, the molecular formulais:
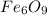
Regards!