Answer:
M = 0.317 m
Step-by-step explanation:
From the given information:
Using the following formula:, the molality M is:
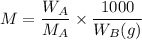
where;
M = molality
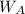 = mass of the solute = 27.1 g
= mass of the solute = 27.1 g
 = molar mass of the solute = 342.8
= molar mass of the solute = 342.8
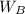 = mass of the given solvent = 250 g
= mass of the given solvent = 250 g
∴
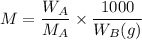
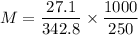
M = 0.317 m