Answer:

Step-by-step explanation:
Since we are given the mass, temperature, and specific heat, we should use the following formula to calculate heat energy.
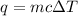
We have 375 grams of water, the specific heat of water is 4.184 J/g ° C, and the temperature is raised 25 degrees Celsisus. Therefore:
- m= 375 g
- c= 4.184 J/g °C
- ΔT= 25°C
Substitute the values into the formula.
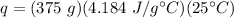
Multiply the first two values together. The units of grams will cancel.
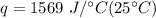
Multiply again. This time, the degrees Celsius cancel, so we are left with only the units of Joules.
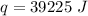
39,255 Joules of heat must be absorbed by the water.