Answer:
8.68 L is the new volume
Step-by-step explanation:
You use Boyle's law for this.
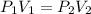
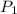 = first pressure
= first pressure
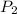 = second pressure
= second pressure
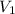 = first volume
= first volume
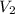 = second volume
= second volume
Convert pressure from atm to mmHg (use same units):
5.97 x 760 = 4537.2 -> 4.54 x 10³
...maintain 3 significant figures in calculation, and round as needed...
(4.54 x 10³ mmHg)(2.79 L) = (1460 mmHg)(
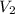 )
)
(4.54 x 10³ mmHg)(2.79 L) / (1460 mmHg) =
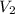 = 8.68 L
= 8.68 L
Hope this helps :)