Answer:
144 seconds
Explanation:
To do solve this question, we must find the LCM (least common multiple) of 12, 16, and 18.
Let's start by prime factorizing (finding the prime factors) all the numbers.

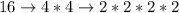
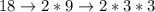
Now, we must find the greatest quantity of each [prime] number and multiply them together to obtain the least common multiple.
Upon further evaluation of the [prime] factors, we can see that we need four 2s and two 3s. Therefore...
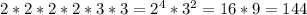
They will be fired together again after 144 seconds.