Answer:
7.19 g of Fe2O3 will produce 0.092 mole of iron
Step-by-step explanation:
One mole of Fe2O3 reacts with 2 mole of Aluminum to produce one mole of Al2O3 and 2 mole of iron.
Mass of one mole of Fe2O3 = 159.69 g/mol
Thus, 159.69 g/mol of Fe2O3 produces two mole of Fe
7.19 g of Fe2O3 will produce
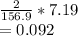 moles of iron
moles of iron