Answer: The space occupied by the gas at 400 torr and
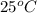 is 250 mL.
is 250 mL.
Step-by-step explanation:
Given:
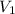 = 250 mL,
= 250 mL,
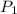 = 800 torr,
= 800 torr,
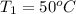
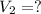 ,
,
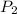 = 400 torr,
= 400 torr,
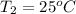
Formula used is as follows.
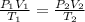
Substitute the values into above formula as follows.
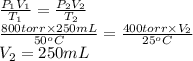
Thus, we can conclude that space occupied by the gas at 400 torr and
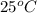 is 250 mL.
is 250 mL.