Answer: The total pressure of given mixture that contains 3 gases is 7.68 atm.
Step-by-step explanation:
Given: Partial pressure of gases is:
Gas A = 4.2 atm
Gas B = 780 mm Hg
Convert mm Hg into atm is as follows.
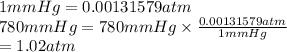
Gas C = 250 kPa
Convert kPa into atm as follows.
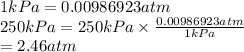
Total pressure is the sum of partial pressures of gases present in the mixture.
Therefore, total pressure of given mixture is calculated as follows.
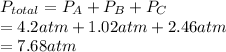
Thus, we can conclude that the total pressure of given mixture that contains 3 gases is 7.68 atm.