Answer:
12.7 L (3 s.f.)
Step-by-step explanation:
Since it is an ideal gas, we can apply the ideal gas law:
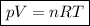
☆ where p= pressure
V= volume
n= number of moles
R= ideal gas constant
T= temperature
Substitute all the given information into the formula:
2V= 1(0.08206)(310)
2V= 25.4386
V= 25.4386 ÷2
V= 12.7 L (3 s.f.)
Further explanation:
The ideal gas constant, R, has different values depending on what units are being used. Two examples are listed below:
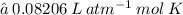
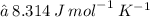
In the above question, we use the value 0.08206 because the pressure was given in atm and the temperature was given in Kelvin. Thus, the unit used for volume is L.