Answer: The reaction
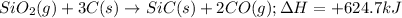 is classified as endothermic: heat is gained by the system.
is classified as endothermic: heat is gained by the system.
Step-by-step explanation:
A process or reaction in which heat is absorbed is called endothermic reaction.
For example, melting of ice is an endothermic process.
For an endothermic reaction the sign of
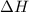 is positive which means heat is gained by the system.
is positive which means heat is gained by the system.
Here, the given reaction is as follows.
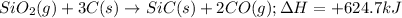
A process or reaction in which heat is released is called exothermic reaction.
For example, combustion reaction, freezing of water etc are exothermic process.
For an exothermic reaction the sign
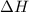 is negative which means heat is lost by the system.
is negative which means heat is lost by the system.
Thus, we can conclude that the reaction
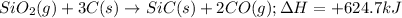 is classified as endothermic: heat is gained by the system.
is classified as endothermic: heat is gained by the system.