Answer: The partial pressure of nitrogen gas if the total pressure is 111.7 atm is 88.243 atm.
Step-by-step explanation:
Given: Mole fraction of oxygen = 0.21
Total pressure = 111.7 atm
It is known that the sum of moles fractions is always equal to 1. So, mole fraction of nitrogen is calculated as follows.
Mole fraction of nitrogen + mole fraction of oxygen = 1
Mole fraction of nitrogen = 1 - mole fraction of oxygen
Mole fraction of nitrogen = 1 - 0.21
Mole fraction of nitrogen = 0.79
Now, formula used to calculate the partial pressure of nitrogen is as follows.
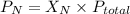
where,
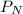 = partial pressure of nitrogen
= partial pressure of nitrogen
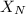 = mole fraction of nitrogen
= mole fraction of nitrogen
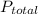 = total pressure
= total pressure
Substitute the values into above formula as follows.
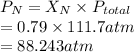
Thus, we can conclude that the partial pressure of nitrogen gas if the total pressure is 111.7 atm is 88.243 atm.